This information is for healthcare professionals based in EU countries only.
Are you a healthcare professional?
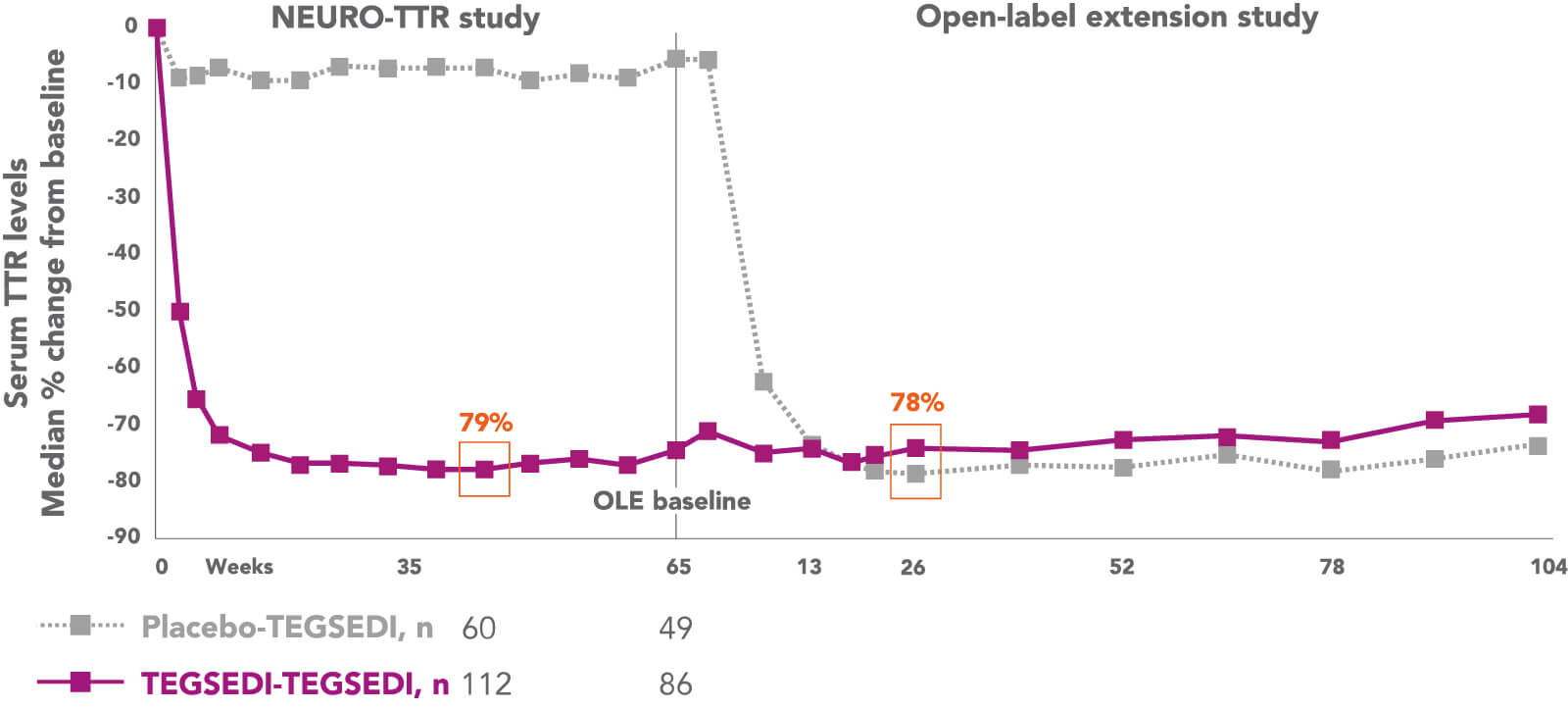
Tegsedi reduces the synthesis of TTR protein in the liver through degradation of TTR mRNA, targeting the disease at its source.1,2
mRNA, messenger ribonucleic acid; TTR, transthyretin.
OLE, open-label extension; TTR, transthyretin
This information is for healthcare professionals based in EU countries only.
Are you a healthcare professional?